
Our genetic makeup has a significant role in health and disease. In this blog, we will explore how well we understand the genetic components of rare disease, and what is being done to improve diagnosis and treatment outcomes for patients.
The importance of genome sequencing
Genome sequencing has become an increasingly useful tool in healthcare. Since the introduction of next generation sequencing, researchers can now see a patient’s entire genetic information within 24 hours.1 Whole genome sequencing enables researchers to analyse the entire genome to identify specific genetic causes of rare conditions which can be targeted to develop tailored diagnostic and treatment strategies.
In our 2022 blog on rare disease, we followed the 100,000 Genomes Project to understand how whole genome sequencing has the potential to revolutionise healthcare, especially for patients with rare disease.
Analysis of data from the 100,000 Genomes Project highlighted the value of genomic data in healthcare; a world-first pilot study demonstrated that whole genome sequencing led to a new diagnosis for 25% of participants.2 Furthermore, a largest of its kind study in 2024 used data from the 100,000 Genomes Project alongside routine clinical data to generate a more in-depth understanding of cancer. The study identified genetic changes which could inform the patient’s treatment plan in 90% of brain tumours.3
The collation of genomic and clinical data to produce a detailed picture of an individual’s health (which can then be analysed as part of a larger group) is not a new concept. UK Biobank has been tracking the health data of 500,000 participants since 2006. Alongside whole genome sequencing, additional participant health data is recorded such as imaging, activity monitoring, questionnaire responses and patient samples including blood and saliva.4 With open access to researchers across the globe, the UK Biobank database is a valuable tool that could change the way we diagnose and treat certain diseases.
Since approximately 80% of rare conditions have a genetic cause,5 rare disease is a key area that can be targeted and better understood by the analysis of genomes from databases, leading the way for tailored methods of treating rare disease.
Establishing a good genetic understanding from birth
Following the success of the 100,000 Genomes Project, the NHS announced the Generation Study in October 2023, which aims to better understand how genome sequencing could be used in the future to screen babies for a wider range of rare conditions. The project aims to perform full genome sequencing on 100,000 newborns by March 2025.6
With 75% of rare diseases known to affect children,7 it is hoped that screening newborns will generate a clearer picture of just how many young lives are affected by rare conditions and provide insight into the genetic variants associated with these conditions.
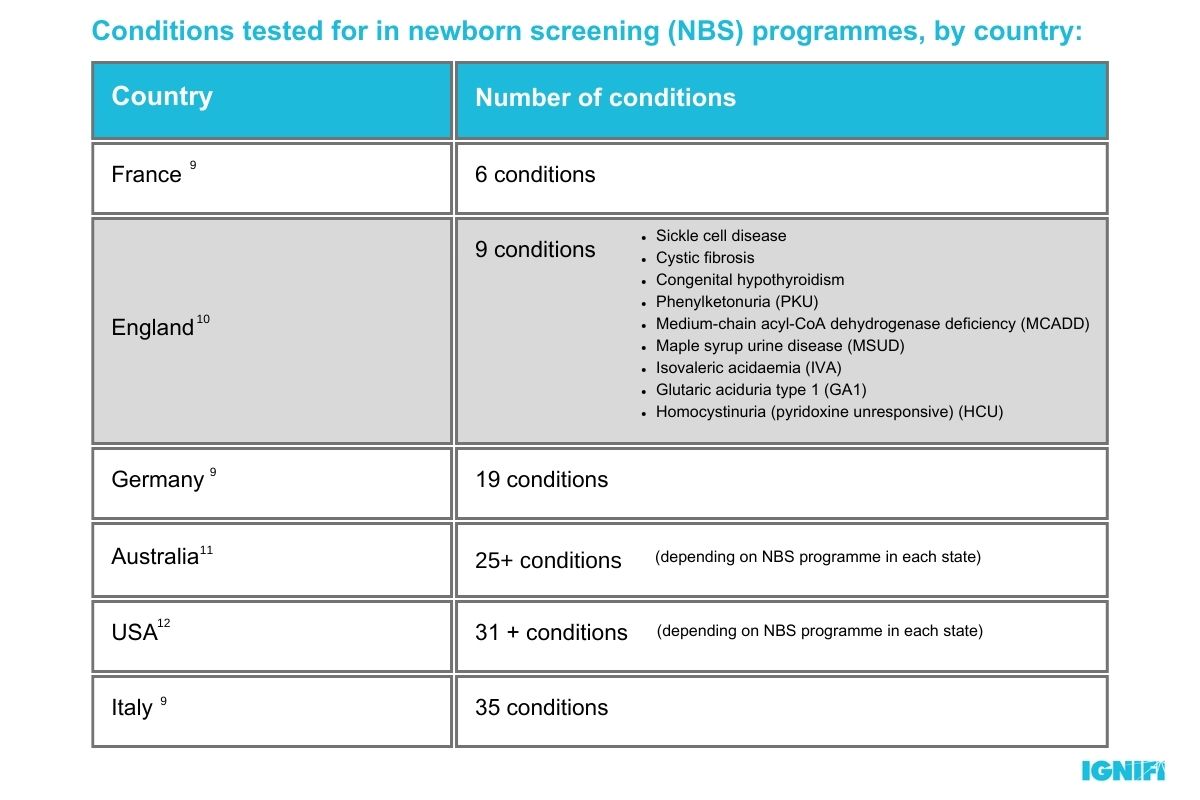
Although the generation study has some real-life benefits for children now and in the future, it is important to acknowledge that whole genome sequencing is a significant step up from the current standard of genetic screening used by the NHS. The standard heel prick, or blood spot test only screens for 9 rare genetic conditions and has not been updated since 20158 despite significant developments in our understanding of the genetic causes of diseases such as Spinal Muscular Atrophy (SMA).
SMA is an inherited rare disease, which leads to a loss of motor neuron function and symptoms such as muscle wasting, weakness and paralysis. Without significant ventilatory support, historically only 8% patients with the most severe type of SMA (SMA1) survive up to 20 months of age.13
Treatment advancements targeting the genetic causes of SMA have recently become available to NHS patients, marking significant investment into rare disease. New data has demonstrated the efficacy of these treatments, as 73% of children with SMA1 in the UK are now older than 2 years.13
As a progressive disease, early diagnosis and treatment of SMA before symptoms appear or worsen can have a major impact on patient outcomes.14 Unlike other European countries, there is no standard newborn screening for SMA in the UK. This can slow diagnosis and mean that potentially-life changing treatments are not reaching SMA sufferers at the earliest opportunity.
Approximately 30% of rare disease patients sadly die before the age of 5,7 therefore fast and accurate diagnosis can have a significant impact on patient outcomes. Projects like the Generation Study will provide key data about the influence of genetics from birth to drive the development of more comprehensive screening methods for rare diseases, so that the right patients are identified and targeted with effective treatment, earlier.
How are rare diseases managed in adulthood?
Though less common, rare diseases also affect adults. Alongside adulthood-onset rare diseases such as Huntington’s disease, the proportion of patients with childhood-onset rare diseases who are surviving into adulthood is increasing, with nearly 16% suffering from haematologic disease.15 The need for effective treatment for rare blood conditions has led to significant developments in treatment options, driven by an improved understanding of their genetic causes.
Hereditary Angioedema (HAE) is a potentially life-threatening condition, where patients experience unpredictable periods of swelling that commonly affects the limbs, face and airway. In recent years, researchers have developed a more in depth understanding of the inflammatory pathways involved in the condition. The blood protein, C1 inhibitor was established to play a key role in these pathways, leading to the development of targeted treatments such as plasma donations that can help regulate the quantity and quality of C1 inhibitor in the blood to manage symptoms.16
The push to replace older and less effective HAE treatments such as androgens has been supported by further genetic research into the disease. A new CRISPR-based genome therapy has shown promising results by targeting one of the pathways that is regulated by the C1 inhibitor, to effectively reduce angioedema attacks.17 This research highlights how an in-depth genetic understanding of disease can enable further pathways to be studied and targeted, for effective treatment of patients who may have uncommon mutations.
Furthermore, HAE is one of the conditions being screened for in the Generation Study.18 Genetic screening for rare hematologic diseases such as HAE from birth could lead to patients being treated earlier for their condition, for better condition management and quality of life.
HAE, like many other rare diseases doesn’t have a single genetic cause. The purpose of genetic research is to identify a range of specific factors involved in rare diseases, made possible by the increasingly in-depth data available from sequencing projects like the 100,000 Genomes Project and UK Biobank. Once disease related factors are identified, treatments can be developed and diagnostic tests implemented so that treatment can have the greatest patient reach.

“For patients living with a rare disease, ending the diagnostic odyssey can be a huge comfort to them. But unfortunately, this is only the beginning of their journey. Many rare disease patients require unique treatments which are tailored to their specific genetic mutation; an approach which is becoming more accessible due to the advancements in genetic testing.”
In our next blog, we will look at how rare disease treatment can be personalised beyond genetics, and understand why these conditions need to be considered on a unique individual basis from drug development to patient treatment plans.
To find out more how IGNIFI can support your rare disease brand click here.








